This overview examines the key biological pathways through which herbal detoxification mechanisms influence liver function, cellular resilience, and metabolic recovery.
Estimated Read Time: 17–21 minutes
Introduction
Herbal detoxification mechanisms describe the biochemical pathways by which plant-derived compounds support the processing, biotransformation, neutralization, and elimination of endogenous and exogenous toxins. These mechanisms engage hepatic Phase I and Phase II detoxification, glutathione synthesis, antioxidant buffering, mitochondrial energy systems, gut–liver axis communication, lymphatic clearance, and autophagic cellular recycling.
The liver remains the central organ in detoxification physiology, but detoxification is not a single-pathway process. Instead, it represents an integrative network involving oxidative metabolism, conjugation pathways, mitochondrial ATP generation, bile production, intestinal microbial balance, endocrine regulation, and immune signaling. When functioning optimally, these processes support resilient metabolic function, cellular homeostasis, hormonal balance, cognitive clarity, and inflammatory stability.
Disruptions in detoxification pathways—due to dysbiosis, mitochondrial dysfunction, chronic inflammatory activity, environmental toxins, medication burden, endocrine imbalances, or metabolic disease—contribute to toxin accumulation, oxidative stress, impaired hepatic function, neuroinflammation, and systemic metabolic overload.
Botanical compounds rich in polyphenols, flavonoids, sulfur molecules, lignans, terpenoids, and glucosinolates interact with detoxification biology in measurable ways, providing support for antioxidant defense, hepatocyte resilience, mitochondrial stability, conjugation pathways, and systemic clearance.
1. Phase I Detoxification: Cytochrome P450 Regulation
Phase I detoxification introduces functional groups onto toxins, pharmaceuticals, metabolic waste products, and environmental chemicals through oxidation, reduction, and hydrolysis reactions. These transformations are largely mediated by the cytochrome P450 enzyme family, particularly CYP3A4, CYP2E1, CYP1A1, and CYP1A2 (1).
Phase I activation produces reactive intermediates that require robust antioxidant support. Herbs that influence Phase I activity typically do so by modulating—not overstimulating—CYP expression, protecting hepatocytes while preserving enzymatic detoxification capacity.
Key Botanicals Supporting Phase I
Milk Thistle (Silybum marianum)
Silymarin complexes stabilize hepatocyte membranes and modulate CYP3A4 and CYP2E1 activity, reducing the production of toxic intermediates (2). Milk thistle increases mitochondrial stability, supports glutathione levels, and protects against alcohol- and toxin-induced liver injury (3).
Curcumin (Curcuma longa)
Curcumin influences CYP1A1 and CYP1A2 activity (4), while significantly reducing oxidative stress through inhibition of NF-κB and pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6 (5).
Green Tea Catechins (EGCG)
Green tea catechins modulate Phase I enzyme expression and provide antioxidant buffering that protects hepatocytes during toxin metabolism (6). EGCG also stabilizes mitochondrial function and reduces oxidative damage induced by reactive intermediates (7).
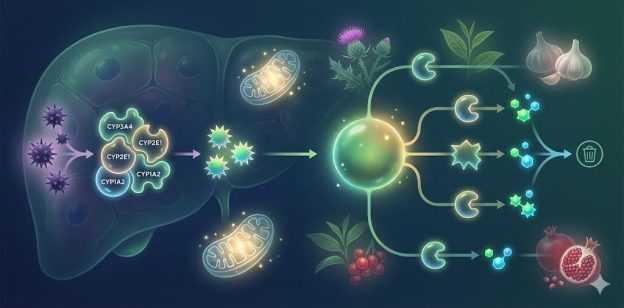
2. Phase II Detoxification: Conjugation & Glutathione Pathways
Phase II detoxification renders Phase I intermediates water-soluble for elimination through conjugation reactions including glucuronidation, sulfation, methylation, acetylation, amino acid conjugation, and glutathione conjugation.
Insufficient Phase II activity leads to accumulation of toxic metabolites and oxidative stress.
2.1 Glutathione Production & Recycling
Glutathione is the primary antioxidant and conjugation molecule for Phase II detoxification. It plays essential roles in neutralizing reactive oxygen species, processing heavy metals, and supporting immune function (8).
Herbs Enhancing Glutathione Pathways:
2.2 Sulfation & Methylation
Botanical compounds influence methylation and sulfation, essential for hormonal detoxification and xenobiotic clearance.
Holy basil, green tea, and turmeric modulate methylation enzymes and reduce oxidative load, supporting detoxification of estrogens, neurotransmitters, and metabolic byproducts (13, 14).
2.3 Glucuronidation
Glucuronidation conjugates toxins, medications, bilirubin, and hormones.
Herbs supporting glucuronidation include:
These botanicals increase UGT activity and reduce enterohepatic toxin recirculation (15).
3. Antioxidant Defense & ROS Neutralization
Detoxification inherently produces oxidative stress. Without adequate buffering, ROS damage mitochondrial membranes, proteins, and DNA.
Herbal antioxidants help maintain redox equilibrium.
3.1 Polyphenol-Dense Botanicals
Herbs with high polyphenol content reduce ROS accumulation and stabilize Phase I/II enzymes.
Examples include:
Plants growing in harsh climates, such as Canadian boreal species, concentrate polyphenols due to environmental adaptation, providing heightened antioxidant potential (17).
3.2 Liver-Protective Antioxidants
Schisandra lignans
Decrease lipid peroxidation and improve mitochondrial respiration (18).
Astragalus polysaccharides
Reduce oxidative stress and support immune-hepatic communication (19).
Gingerols
Protect hepatocytes from oxidative injury and improve mitochondrial enzyme efficiency (20).
4. Mitochondrial Resilience & Energy-Dependent Detoxification
Botanical compounds enhance mitochondrial efficiency, protect mitochondrial membranes, and support NADPH recycling.
4.1 Mitochondria-Supportive Herbs
Rhodiola rosea
Improves mitochondrial ATP production under metabolic stress (21).
Schisandra chinensis
Enhances mitochondrial respiration and stabilizes oxidative environments within hepatocytes (22).
Holy basil
Supports mitochondrial antioxidant enzymes and reduces cortisol-related metabolic strain (23).
Lion’s Mane (Hericium erinaceus)
Promotes mitochondrial stability in neural cells and supports neuro-metabolic detoxification (24).
4.2 NADPH & Glutathione Recycling
Botanicals help maintain NADPH availability required for:
Flavonoids and polyphenols are especially effective at stabilizing intracellular NADPH levels (25).

5. Gut–Liver Axis: Bidirectional Regulation of Detoxification
Intestinal permeability permits translocation of lipopolysaccharides (LPS), amplifying hepatic inflammation (26). Dysbiosis alters bile acid signaling and interferes with Phase II conjugation pathways.
5.1 Botanical Support for the Gut–Liver Interface
Licorice (DGL)
Strengthens mucosal integrity and reduces translocation of bacterial metabolites (27).
Chamomile
Reduces intestinal inflammation, decreasing hepatic inflammatory signaling (28).
Berberine
Improves microbiota composition, enhances SCFA production, and reduces LPS burden (29).
Ginger & peppermint
Enhance digestive motility and bile flow.
Milk thistle
Promotes bile production and supports hepatic elimination through improved bile synthesis (30).
Gut-derived metabolites significantly affect detoxification efficiency and hepatic inflammation, demonstrating the interconnectedness of digestive and metabolic systems (31).
6. Lymphatic Transport & Interstitial Detoxification
The lymphatic system clears inflammatory proteins, immune complexes, metabolic waste, and cellular debris from tissues. Impaired lymphatic circulation contributes to toxin accumulation and chronic inflammation.
6.1 Lymphatic-Supporting Botanicals
Red Clover (Trifolium pratense)
Promotes lymphatic flow and reduces interstitial congestion (32).
Cleavers (Galium aparine)
Supports removal of metabolic byproducts through lymphatic drainage (33).
Calendula
Facilitates lymphatic movement and tissue repair.
Astragalus
Supports lymphatic-immune coordination (34).
Reishi mushroom (Ganoderma lucidum)
Regulates immune-lymphatic activity through beta-glucans (35).
7. Autophagy: Intracellular Detoxification Mechanisms
Autophagy removes damaged organelles, misfolded proteins, and dysfunctional mitochondria while supporting cellular recycling and metabolic renewal.
Autophagic activity declines with age, inflammation, metabolic disease, and mitochondrial dysfunction.
7.1 Autophagy-Activating Botanicals
Curcumin
Activates AMPK, stimulates autophagic clearance, and improves cellular stress responses (36).
Ginseng
Enhances autophagy through PI3K/Akt modulation (37).
Green Tea Catechins
Promote mitochondrial biogenesis and intracellular detoxification (38).
Rosemary diterpenes
Support autophagy, cellular repair, and antioxidant defenses (39).
Autophagy plays a critical role in the detoxification of neural tissues, metabolic organs, and energy-dependent systems.
Clinical Implications of Herbal Detoxification
Effective detoxification contributes to:
Herbal contributions to detoxification represent an evidence-based approach to supporting systemic function across multiple organ systems.
Safety Considerations
Botanicals influencing detoxification pathways require clinical awareness due to potential interactions with medications (especially CYP-modulated drugs), gallbladder disorders, pregnancy, hepatic impairment, and immunosuppressive therapy.
Herbal detoxification mechanisms should be contextualized within individualized medical assessment.
Conclusion
Herbal detoxification mechanisms integrate hepatic biotransformation, antioxidant defense, mitochondrial resilience, microbial balance, lymphatic function, and cellular recycling. Botanical compounds provide multifaceted biochemical support for detoxification processes, contributing to inflammation regulation, metabolic recovery, and long-term physiological stability.
These interconnected pathways underscore the complexity of detoxification physiology and the potential role of botanicals in supporting safe, evidence-based metabolic repair.
📚 References
