🌿 The Inflammation–Microbiome Axis: How Herbal Compounds Influence Gut–Immune Signaling in Chronic Disease
Estimated Read Time: 10–12 minutes
Introduction
The past decade has radically transformed our understanding of human biology. The gut is now viewed not merely as a digestive organ but as a dynamic ecosystem deeply influencing immunity, inflammation, metabolism, cognition, and chronic disease. At the center of this system is the gut microbiome, a community of trillions of microorganisms that function as a metabolic and immunological organ.
One of the most important discoveries in modern medicine is the inflammation–microbiome axis—the bidirectional communication between gut microbes and the immune system. Disturbances in this axis are now linked to:
At the same time, modern research increasingly reveals that herbal compounds directly modulate the microbiome and its inflammatory signaling pathways. Many traditional herbs long used for digestion, inflammation, and immunity are now shown to act through microbiome-mediated mechanisms.
This article provides a rigorous, evidence-based exploration of how herbal medicine influences the gut–immune–inflammation axis—bridging traditional botanical knowledge with modern immunology and microbiome science.
1. The Microbiome–Inflammation Axis: A Scientific Overview
1.1 Microbes as Immune Controllers
Approximately 70% of the immune system resides in the gut, where immune cells constantly interact with microbial metabolites, antigens, and structural components [1]. Healthy microbial populations help regulate:
Disruption in microbial balance (dysbiosis) can trigger chronic inflammation through multiple pathways.
1.2 Dysbiosis and Chronic Inflammation
Dysbiosis promotes inflammation by:
This systemic immune activation is clinically associated with:
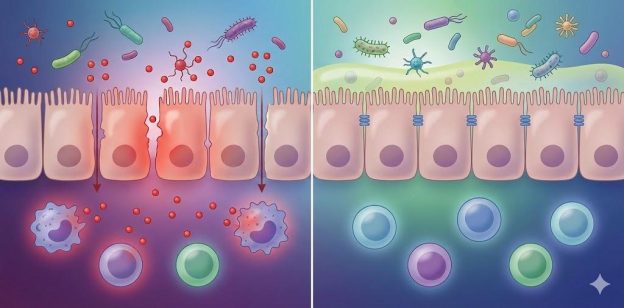
1.3 Gut Barrier Breakdown: A Key Driver of Systemic Illness
The gut lining (mucosal barrier) regulates what enters the bloodstream. When tight junctions weaken—through stress, antibiotics, infections, or poor diet—microbial toxins enter circulation and fuel inflammation.
This process contributes to:
Many herbal compounds support mucosal healing, SCFA production, and microbial balance, restoring barrier integrity.
2. How Herbal Compounds Influence the Microbiome
Herbal compounds affect the microbiome in at least five major ways:
2.1 Acting as Prebiotics
Polyphenols and plant fibers feed beneficial bacteria such as Bifidobacteria and Lactobacilli.
2.2 Antimicrobial Modulation (Not Sterilization)
Some herbs suppress harmful species while preserving beneficial populations—known as “selective antimicrobial action.”
2.3 Enhancing Short-Chain Fatty Acid (SCFA) Production
SCFAs (butyrate, propionate, acetate) reduce inflammation, nourish colon cells, and tighten gut junctions.
2.4 Reducing LPS and TLR Activation
Herbal compounds can reduce the inflammatory consequences of microbiome imbalance.
2.5 Supporting Mucosal Healing
Certain herbs increase mucous production, reduce epithelial inflammation, and stabilize tight junction proteins (zonulin, claudins, occludins).
3. Evidence-Based Herbal Modulators of the Microbiome–Inflammation Axis
Below is a detailed review of herbs with strong emerging scientific support for microbiome and immune modulation.
3.1 Berberine (Goldenseal, Barberry, Oregon Grape)
Berberine is one of the most powerful plant compounds affecting the gut microbiome and metabolic inflammation.
Key Mechanisms
Clinical Relevance
Used in studies on:
Caution
Strong herb–drug interaction potential; requires clinical oversight.
3.2 Licorice Root (Glycyrrhiza glabra)
Licorice is traditionally used for gut inflammation, ulcers, and mucosal healing.
Mechanisms
Applications
Useful for:
3.3 Ginger (Zingiber officinale)
Ginger is one of the most researched herbs for digestion and systemic inflammation.
Effects on Microbiome
Anti-Inflammatory Pathways
Clinical Relevance
May support gut motility, nausea, and chronic inflammatory conditions involving gut–immune signaling.
3.4 Chamomile (Matricaria chamomilla)
Chamomile has both gut-calming and anti-inflammatory properties.
Microbiome Effects
Clinical Relevance
A good candidate for:
3.5 Pomegranate Polyphenols (Punicalagins)
Pomegranate tannins reach the colon largely unmetabolized, where gut bacteria convert them into powerful anti-inflammatory metabolites.
Key Benefits
Applications
Proven relevance in metabolic health, inflammatory cascades, and gut barrier support.
3.6 Fireweed (Chamerion angustifolium) — A Canadian Example
Fireweed contains mucilage and antioxidants beneficial for gut restoration.
Scientific Findings
Traditional use aligns closely with modern gastrointestinal research.
3.7 Slippery Elm (Ulmus rubra) and Marshmallow Root (Althaea officinalis)
These herbs are rich in soothing mucilage.
Benefits
Relevant for:

4. Gut–Immune–Brain Connections
The microbiome sends signals to the brain through:
Herbs that reduce gut inflammation or feed beneficial microbes may indirectly support:
This is highly relevant in post-viral fatigue and long COVID, where neuroinflammation and microbiome disruption frequently coexist.
5. Herbs and the Gut Barrier (Intestinal Permeability)
Herbs supporting barrier integrity include:
Herb | Mechanisms |
|---|---|
Licorice (DGL) | Tight-junction repair, mucous layer strengthening |
Slippery elm | Barrier coating, microbiome support |
Marshmallow root | Anti-inflammatory, mucilage-mediated healing |
Turmeric | Tight-junction stabilization; NF-κB inhibition |
Ginger | Reduction in LPS translocation |
Pomegranate | SCFA enhancement; barrier reinforcement |
Damage to the gut barrier is linked to:
Supporting the barrier is therefore essential in integrative health.
6. Safety Considerations
Herbal medicine is safe when properly selected and personalized; unsafe when used casually or without guidance.
7. Future Directions in Herbal–Microbiome Research
Emerging research trends include:
7.1 Post-Viral Illness & Long COVID
Dysbiosis and LPS elevation are increasingly implicated in long COVID pathophysiology.
7.2 Microbiome-Derived Metabolites
Herbal polyphenols may gain therapeutic relevance through metabolites produced by gut bacteria (e.g., urolithins from pomegranate).
7.3 Herbal Synergy & Microbiome Adaptation
Combined herbal formulas may create shifts in microbial metabolites greater than individual herbs.
7.4 Personalized Herbal–Microbiome Medicine
AI-driven microbiome profiles may help match herbs to individual inflammatory phenotypes—a future direction aligned with iHerbMed’s long-term vision.
Conclusion
The gut microbiome is now recognized as a central regulator of inflammation, immunity, and chronic disease. The emerging field of herbal–microbiome interactions reveals that many traditional botanicals exert their benefits not only through direct anti-inflammatory actions but also by reshaping microbial ecosystems, strengthening gut barriers, and modulating immune signaling.
Berberine, licorice, ginger, chamomile, pomegranate, fireweed, and mucilaginous herbs offer scientifically grounded pathways to support the gut–immune–inflammation axis. When used responsibly—and integrated with nutrition, sleep, stress management, and medical care—these herbal compounds become valuable components of an evidence-based, whole-body recovery framework.
📚 References
